Smartphones: the Lateral Flow Readers in Your Pockets
Lateral Flow Brings Several Advantages Over Other Forms of Diagnostic Testing
AI and Machine Learning Technology Opening New Doors
Considering Smartphone Technology on Lateral Flow Test Projects
Key Take-Away
Lateral Flow Market 2.0 Series: Part 3
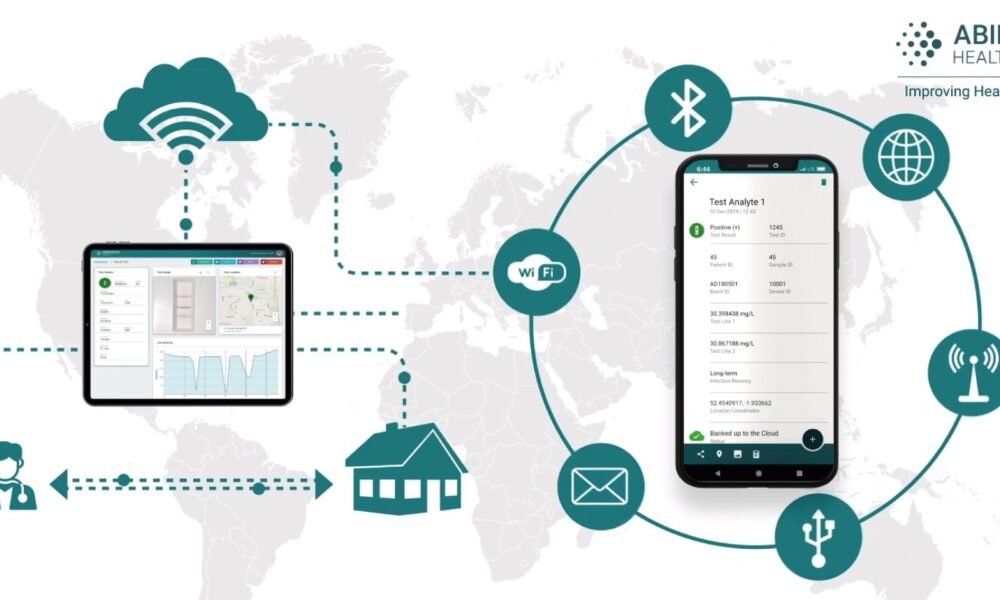
As highlighted in our earlier blog post, Lateral Flow Market 2.0, digital is emerging as a significant theme in lateral flow innovation. One key technology driving this innovation is the emergence of smartphone readers – such as Abingdon’s AppDx® mobile reader – as commercially viable solutions for accurately interpreting lateral flow tests. In this follow up, we expand on why we believe smartphone readers can accelerate and enhance applications of lateral flow, and describe the key considerations when looking to integrate smartphone reader technology into your project or product.
Lateral Flow Brings Several Advantages Over Other Forms of Diagnostic Testing
Lateral flow tests versus other forms of diagnostic testing are timely, scalable, easy-to-use, cost-effective and are playing an increasingly prevalent role in today’s health landscape. On the flip slide, their decentralised nature can present a challenge to ensure correct test operation and result interpretation. Mobile devices that use only the device’s built-in camera are now able to automate this process and reduce subjectivity in the result; a common issue when reading with the human eye.
AI and Machine Learning Technology Opening New Doors
Current artificial intelligence (“AI”) and machine-learning technology are major factors behind this advancement, providing both real-time and extremely accurate reads. With machine-learning, reader software can also be quickly adapted to a new manufacturer’s test. This opens a world of potential in impactful areas, such as disease monitoring, integrated clinical pathways, and supply chain analytics.
As an illustration of recent progress, our own AI-powered app, AppDx®, was able to achieve 98+% sensitivity and specificity on a blood-based test for COVID-19 antibodies. AppDx® can also be trained to make a semi-quantitative interpretation or read multi-line qualitative assays. In addition to high precision, the interpretation happens completely on-device, with no internet connection required; delivering a result in real-time and a seamless experience for the user. We believe the use of on-device AI is a key differentiator for AppDx®, reducing model serving costs and allowing mobile readers to operate in challenging, resource-constrained environments.
Considering Smartphone Technology on Lateral Flow Test Projects
The potential for smartphone readers is significant, but not without its challenges, and we set out below some of the key areas that should be considered when appraising the integration of smartphone technology into your own lateral flow project.
Research And Development Phase
Any project looking to utilise smartphone reader technology must first undergo a research and development phase. This is where the algorithm is refined to a specific test. This requires a range of positive and negative test result images to build the data set and allow the algorithm to “learn.” The target is a refined algorithm, with algorithm performance at the required levels. For Abingdon’s AppDx® software, this requires around 500-1,000 tests. To streamline this process, we have developed cloud-based data collection tools that can be used in laboratories or industrial facilities and automatically connect to our infrastructure. For AppDx® the output of this training phase is a test-specific Research & Development (“R&D”) version of the app, where the performance of the algorithm can be further evaluated and additional data gathered, if necessary.
Pricing Model
It is important to choose a smartphone reader solution that provides a pricing model that meets your requirements. At Abingdon our pricing model consists of a fee for the R&D phase to account for its experimental nature, and following successful evaluation of the R&D app, an annual licence fee. This contrasts with other pricing models in the lateral flow industry; for example, some operators offer a “pay per clicks” model. One additional approach we offer is to provide a smartphone Software Development Kit (“SDK”) that has been designed to allow easy integration of AppDx® into a customer’s existing app, or allows the customer to develop its own app independently and plug the SDK (reader) into this.

Intellectual Property
Another important consideration is the patent landscape for smartphone readers. At Abingdon, we have a long and successful history of developing reader technology and have invested in a strong intellectual property (“IP”) position to support reader devices. The IP is in the form of proprietary source code, internal engineering designs, know-how and patents. This includes patents specifically for AppDx® and we continue to file UK and international patents against the technology.
Regulatory Approach Plan
One key aspect to consider at the outset of a project is the regulatory approach plan. The approach will depend on the market being targeted and the product being used alongside the smartphone reader. If we consider the clinical self-test market, it is interesting to note that the FDA advises, and actually encourages, sponsors interested in developing tests for home-use to develop a digital companion to the test, i.e., a mobile app, which could, among other things, facilitate contact with care and also provide educational content for the user. In general, as part of the lateral flow assay development process, it is important to incorporate the smartphone reader into the validation and verification process, as well as external evaluations and clinical trials. Planning at the outset is essential here, to ensure the smartphone app development process runs in parallel with an assay development process. Abingdon’s regulatory team can support building a regulatory approach plan for the overall solution; the smartphone app and the lateral flow test.
The Key Takeaway
The market for lateral flow is undergoing a second wave of innovation, in the wake of COVID-19 and amid increased awareness of the advantages of this class of diagnostic devices. This is growing the range of applications to which lateral flow can be applied.
To truly unlock those applications, we believe smartphone reader technology – backed by recent advances in AI and machine learning – has an important role to play. Abingdon Health is proud to be at the forefront of this technology and we look forward to working with new partners on integrating the AppDx® solution into your projects.
Please contact us at [email protected] to discuss how we can help.

