There are some common myths that lateral flow devices are inflexible and not suitable or flexible enough for an ever demanding marketplace. Therefore, we dispel some of these common myths about lateral flow devices (LFDs).
Myth - LFDs can only be qualitative.
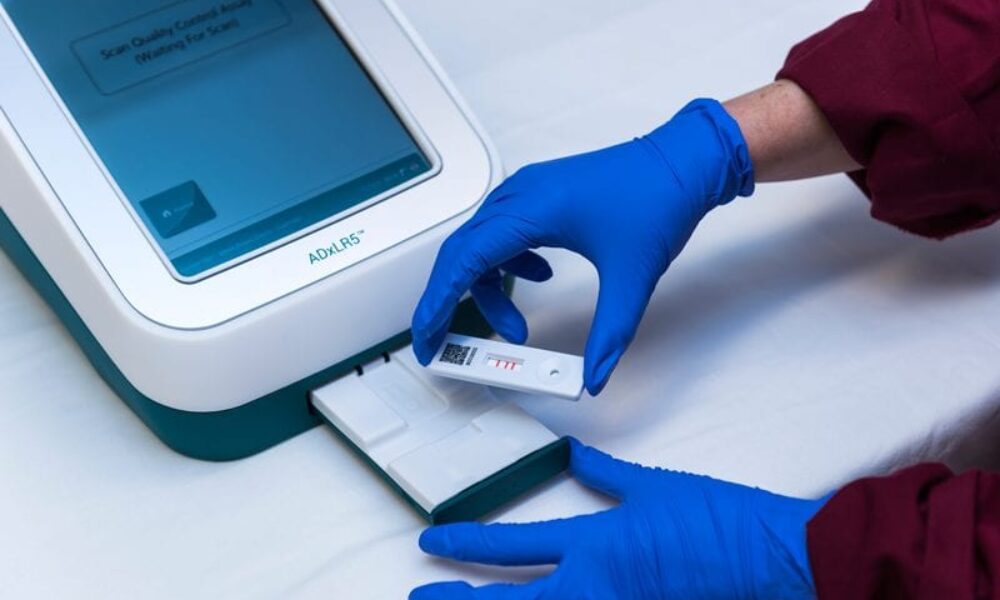
In the early days, LFDs were predominantly qualitative assays but improvements in reagents, component materials, and reader technologies have meant fully quantitative results are achievable – not everyone is aware of this development!
The need for real-time results to enable informed decision making, across many sectors, can be realised with lateral flow, whether that be a qualitative or quantitative assay.
Myth - The accuracy of LFDs do not meet laboratory requirements.
Selecting the reagents capable of meeting the required assay performance is the critical starting point to all our projects. With new developments in reader technology, a lateral flow test can match the sensitivity of an ELISA assay. In addition, any results produced by a reader can be stored and integrated with data management systems.
Abingdon Health spoke at a webinar which directly challenged the myth about lateral flow inaccuracy.
Myth - LFDs are not flexible enough to meet growing diagnostic challenges.
It’s always important to think through the test procedure and purpose, i.e. how the sample will be collected, where and by whom the assay will be performed, so the right development choices are made. To maximise time or improve workflow LFDs can be multiplexed by having multiple lines per strip and multiple strips per device. There may also be applications best suited to a low-cost dipstick style device which is packaged in bulk rather than individual foiled wrapped cassettes. So yes, LFDs are flexible and can be developed to meet the needs of a wide range of applications.
If you would like to speak with us about the capabilities of lateral flow devices please contact us on +44 (0) 1904 406050 or [email protected].