How To Select The Most Appropriate Sample Pad

Heightened Sensitivity Opening New Doors
The Right Components Are Key
What Are The Options?
To Pre-Treat A Sample Pad, Or Not?
Key Take-Away
The COVID-19 pandemic has resulted in the democratisation of Lateral Flow Immuno-Assay (LFIA) technology, leading to the expansion of rapid test kits for various applications. The focus remains on increasing the sensitivity, accuracy, and complexity of these tests. While the detection and quantification of infectious disease markers continue to be a popular development in LFIA, this easy, quick, and affordable testing method is also used in other applications such as wellness, food control, and substance abuse monitoring.
In this guest blog, Frédéric Bébien, Product Development Manager [Lab & Life Science] at Ahlstrom – the leading global provider of sustainable fibre-based specialty materials for use in Filtration, Food & Consumer Packaging, Healthcare, Building Materials and Technical Materials – discusses advances in sample pad materials available for use in LFIA development, their various merits and other key considerations.
Heightened Sensitivity Opening New Doors
Continuous improvement in LFIA sensitivity has made it possible to work with new sample types with low analyte concentration. This simplifies the collection and preparation process, leading to a new generation of common tests, like pregnancy tests, that detect from whole blood or saliva. These tests are easier, less invasive, and more convenient for the end user compared to other screening methods.
The Right Components Are Key
In the science of LFIA, selecting the right components for assay design, development, and transfer to production-scale is essential. This involves selecting the appropriate reagents and solid materials, like membranes and fibre-based materials pads used as sample pads, conjugate pads, and absorbent pads.
As the leader in the manufacturing of key components for rapid diagnostic tests, Ahlstrom has developed throughout the years a comprehensive portfolio of sample pads for use in LFIA development and manufacturing, capable of responding to differentiated needs. Choosing the most appropriate materials for your assay and its intended application, is thus crucial for achieving reliable and consistent results.
What Are The Options?
When it comes to selecting fibre-based materials, several important questions must be addressed, such as design, volume, quantitative or qualitative assay, and the manufacturing process. However, the most crucial aspect is to understand the matrix. Once these questions are answered, screening and selecting fibre-based sample pad materials can begin.
For sample pad material selection, several fibre options and dimensions are available, including cotton / cellulose, glass, micro-glass, synthetic, and blend of fibres.
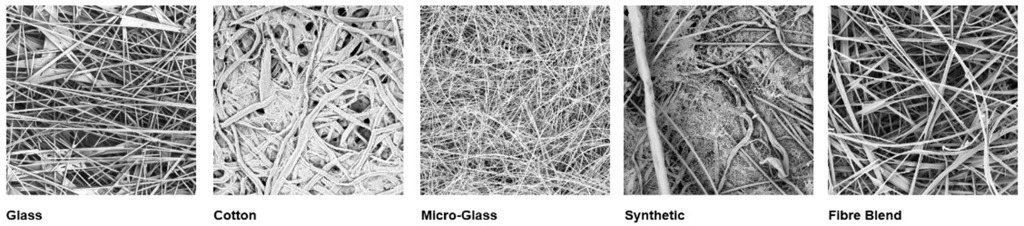
Glass: Glass fibre materials have a more porous structure, making them appropriate for samples that do not require filtration, like viscous and limited volume of samples such as saliva and nasal samples.
Cotton / Cellulose: This sustainable material is hygroscopic and absorbent, ensuring consistency in absorption and uniform distribution of samples onto the conjugate pad.
Micro-Glass: μGlass fibres, thanks to their dimensions and inert features, provide efficient sample filtration of samples like blood, or milk; removing fatty globules or cells that could affect membrane flow and interact with the reading of the test result.
Synthetic: This provides another source of fibre material with a more porous structure; different interaction compared to Glass fibre material may be observed.
Fibre Blend: this material gives a wider pore size distribution than pure fiber and it is ideal for blood filtration to limit pressure in the fiber pores, avoiding Hemolysis, the destruction of red blood cells.
To Pre-Treat A Sample Pad, Or Not?
In addition to the choice of fibre-based material structure, sample pad pre-treatment is also a critical consideration. Treating the sample pad with chemicals like proteins, detergents, surfactants, and buffer salts can improve its performance. For example, pre-treatment can improve blood separation efficiency, change the pH of the sample, improve the consistency of the conjugate release, and potentially minimise analyte retention for improved lateral flow assay sensitivity.
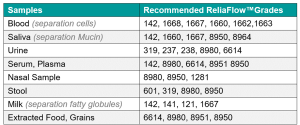
The technology used for pre-treating the sample pad must be carefully designed to achieve uniform treatment with the proper concentration of the chemical, consistent coverage and drying, and no contamination. This leads to improved quality, higher reliability, and better lot-to-lot consistency tests, making it easier to develop and transfer assays to production-scale.
Key Take-Away
It is critical to partner with a reputable, experienced LFIA contract development & manufacturing organisation (CDMO) to ensure you’re presented with the best possible expertise, experience and supply chain access available to ensure product success; from selecting the best quality device materials to ensure optimum test quality and efficacy with your chosen sample type, to guiding you on development and optimisation decisions based on trial data and the latest market and technological advances.
Ahlstrom are proud to be partnered with Abingdon Heath to continuously improve solutions for Diagnostics.

