How Innovation in Labels Is Driving Growing Use of Lateral Flow Technology

An Introduction to Labels
A Growing Range Of Options
So, Which Label Is Right For You?
Key Takeaway
Label selection should be a critical element of any lateral flow development process. In this blog Charlotte Howard, R&D Lead Scientist at leading lateral flow CRO and CDMO Abingdon Health, outlines why innovation in lateral flow is driving the expanding use of lateral flow products and technology across a range of sectors.
An Introduction to Labels
In essence, labels in a Lateral Flow Device (LFD) are the detector moiety which allows visualisation and, if required, quantification of the test result. In effect an LFD is only as good as its label; after all, without it how would one interpret or read the result. Many traditional lateral flow diagnostic devices use gold as the detector and this label typically gives the red/pink test line most of us are familiar with from the COVID-19 lateral flow tests used during the pandemic.
Through innovation we are seeing an increasing range of label options available for use in lateral flow technology, which is increasing the breadth and scope of the useful applications of LFDs. New labels also improve the ability to meet specific customers’ needs; be it aesthetically in their search to make it match their minds eye with defined colours for each sample; for higher contrast and easier visualisation by a lay person (important with the growth in self-testing) or reader; or even sensitivity-wise to ensure that even low levels of sample are detected. As such, the label is one of the most important aspects of development for a lateral flow device.
A Growing Range Of Options
While gold is still the preferred label, different “nanoshapes” are becoming alternatives to improve sensitivity. One option is nanoshells(1) which consist of a silica core surrounded by a gold shell providing an ultrabright label. These can vary in size from 10 – 200nm, which changes the thickness of the shell, altering the resonance so the particle can be tuned to visible or infrared spectra, allowing either visual readout or use of a reader(2). Size also changes the colour, varying from a red-purple colour at 20nm to blue at 150nm. This darker contrast with blue against the white membrane, relative to red, makes it easier for readers to contrast to improve the assay readout.
Nanoshells can have a variety of surfaces including carboxyl, NHS, PEG, PVP etc. which facilitate different reactions. Carboxyl conjugation can be used to facilitate covalent protein binding via EDC/NHS chemistry, ensuring that antibodies/proteins bind successfully via amide bonds.
Alternatively, with the new age of multiplexing coming to the forefront in lateral flow devices, identification of each sample is made simpler for end-users by using nanoparticles with different colours. These can range from red to blue, to green, or even to pink or yellow, depending on your colour preference. Using different colours for your test and control line can make it easier for end-users to confirm if the device is positive or negative with a simple glance.
Others are now offering new labels to the market for improving sensitivity in LFDs, such as Merck who now offer the dyed Estapor® microspheres which are a form of latex. These are offered in a variety of colours where they are dyed internally to avoid changing their surface properties or leaching into aqueous buffers. These microspheres can be provided in a range of conditions, including unfunctionalized – where they have no functional groups, facilitating passive conjugation of larger molecules. However, if you are working with smaller molecules that are less than 10kDa, hydroxyl, carboxyl or chloromethyl modified microspheres are offered.
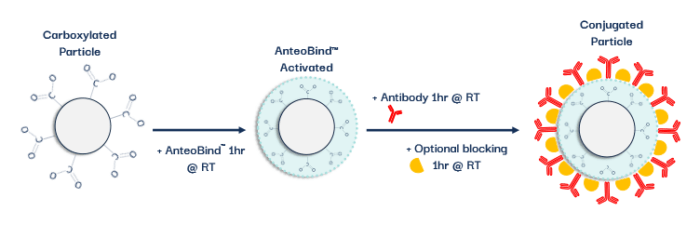
Anteobind™ is new to the market providing an alternative to conventional carboxyl conjugation, making use of a polymeric metal ion complex(3). However, while covalent interactions are used to improve label conjugation, they target specific amino acids with carboxyl groups. As such, sometimes the Fc region or the Fab region can attach to the colloid and, therefore, the orientation is not optimal for exposing the binding site of the antibody resulting in lower sensitivity. The metal-ion complex of Anteobind™ allows a flexibility of the binding molecules without breaking away from the surface, focusing on binding to the histidine cluster in the Fc region of the antibody resulting in a preferential orientation of the antibody and allowing more access for sample interaction, thereby increasing sensitivity.
So, Which Label is Right For You?
With this range of options, the importance of defining the Product Requirement Specification (“PRS”) at the outset of a lateral flow test development project is critical. This document will define the essential and desirable elements that will be critical in building a product that meets, for example, the clinical requirements; user requirements; and also the requirements for scalable manufacture.
Preparation of the PRS is a key first step in Abingdon’s product development pathway with customers and this PRS will inform crucial decisions in component selection, including selecting the appropriate label for use in your lateral flow assay. There is not a ‘one size fits all’ option… At Abingdon have a broad network of label providers and can work with you, leveraging our experience and expertise, to determine the most appropriate label type and provider. This ensuresg you have access to cost-effective solutions that meet your technical requirements, with a supplier that can offer surety of supply; not just for development, but for the long-term when the product transitions into manufacture.
Key Takeaway
Innovation is driving an increase in the range of labels available for lateral flow development. This is also expanding the potential use of lateral flow technology as, for example, new labels are driving the ability for lateral flow technology to become more sensitive; pushing the boundaries of lateral flow technology, and therefore broadening the range of potential applications.
Choice of label will depend on the product requirements and Abingdon Health, as a fully integrated CRO and CDMO, can support you in deciding which label and which supplier meets your specific requirements. Going forward, we also foresee the range of available labels increasing, allowing innovative LFT development to build improved and differentiated lateral flow products in existing markets as well as new ones.
For more information on how Abingdon Health can support your product’s journey from idea to commercial success, please contact a member of our team to discuss this subject in confidence.
References
- Muñoz‐Ortiz, T.; Hu, J.; Ortgies, D. H.; Shrikhande, S.; Zamora‐Perez, P.; Granado, M.; González‐Hedström, D.; de la Fuente‐Fernández, M.; García‐Villalón, Á. L.; Andrés‐Delgado, L.; Martín Rodríguez, E.; Aguilar, R.; Alfonso, F.; García Solé, J.; Rivera, P.; Jaque, D.; Rivero, F. Molecular Imaging of Infarcted Heart by Biofunctionalized Gold Nanoshells. Adv. Healthcare Mater. 2021, 2002186.
- Behrouzi K and Lin L. Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleoclapsid proteins. Biosensors and Bioelectronics. 2022; 195:113669:
- Karimian, Tina, et al. A Simplified and Robust Activation Procedure of Glass Surfaces for Printing Proteins and Subcellular Micropatterning Experiments. MDPI, 2022.

