LFA Design: Mastering the Art of Choosing Gold

The Brilliance of Gold Nanoparticle
Demystifying Gold Conjugates
Key Takeaways
Gold Nanoparticles & Gold Antibody Conjugates for Lateral Flow Immunoassays
In this guest blog, Noan Nivarlet, Managing Director of Nano Flow EU – a division of Belgian food-biotech company UniSensor, and a leading manufacturer-supplier of sustainable, high-quality gold nanoparticles and conjugates – weighs up the power of leveraging gold nanoparticles and gold conjugates for optimal sensitivity and accuracy in lateral flow immunoassay development projects.
Gold nanoparticles have emerged as the most widely utilised antibody labelling system in lateral flow immunoassays. This popularity can be attributed to several key factors. Firstly, gold nanoparticles exhibit exceptional stability and biocompatibility, ensuring long-term performance and minimising potential adverse effects. Additionally, their unique optical properties, such as strong surface plasmon resonance enable easy visual detection, enhancing the assay’s simplicity and accessibility. Moreover, gold nanoparticles can be easily functionalised with antibodies, providing high binding capacity and sensitivity.
These advantageous characteristics make gold nanoparticles the preferred choice for efficient and reliable antibody labeling in lateral flow tests.
The Brilliance of Gold Nanoparticles
When selecting gold nanoparticles for lateral flow immunoassays, there are several crucial factors to consider.
- The nanoparticle size directly affects the assay’s sensitivity, and the optimal size may vary depending on the test format (sandwich vs. competitive). While 20nm gold colloids may be suitable for competitive assays, larger sizes should be tested for sandwich assays. In general, starting with 40nm gold particles is a good compromise, regardless of the test format.
- Particle stability is vital to prevent aggregation and maintain consistent performance. Using spherical particles with smooth edges and uniform shapes, helps prevent gold particle aggregation. These highly spherical particles, such as those offered by Nano Flow, provide antibodies (or other biomolecules that will be conjugated) with a maximum contact surface area, resulting in excellent sensitivity, optimal binding, consistent antibody-antigen kinetics, homogeneous coverage, and high stability. [The image below shows differences in gold nanoparticle shapes under the microscope, in comparison with Nano Flow’s product.]
- Additionally, strong and distinguishable optical properties are important for visual detection or quantification of results in lateral flow immunoassays.
- And finally, cost-effectiveness is crucial for scalable production.
Considering these parameters ensures the selection of gold nanoparticles that can reliably and accurately deliver results in lateral flow immunoassays.

Demystifying Gold Conjugates
Attaching antibodies to gold nanoparticles is a relatively straightforward process, but achieving gold conjugates with optimal sensitivity, stability, and reproducibility poses a greater challenge.
Various strategies can be employed to attach antibodies or biomolecules to the surface of gold nanoparticles.
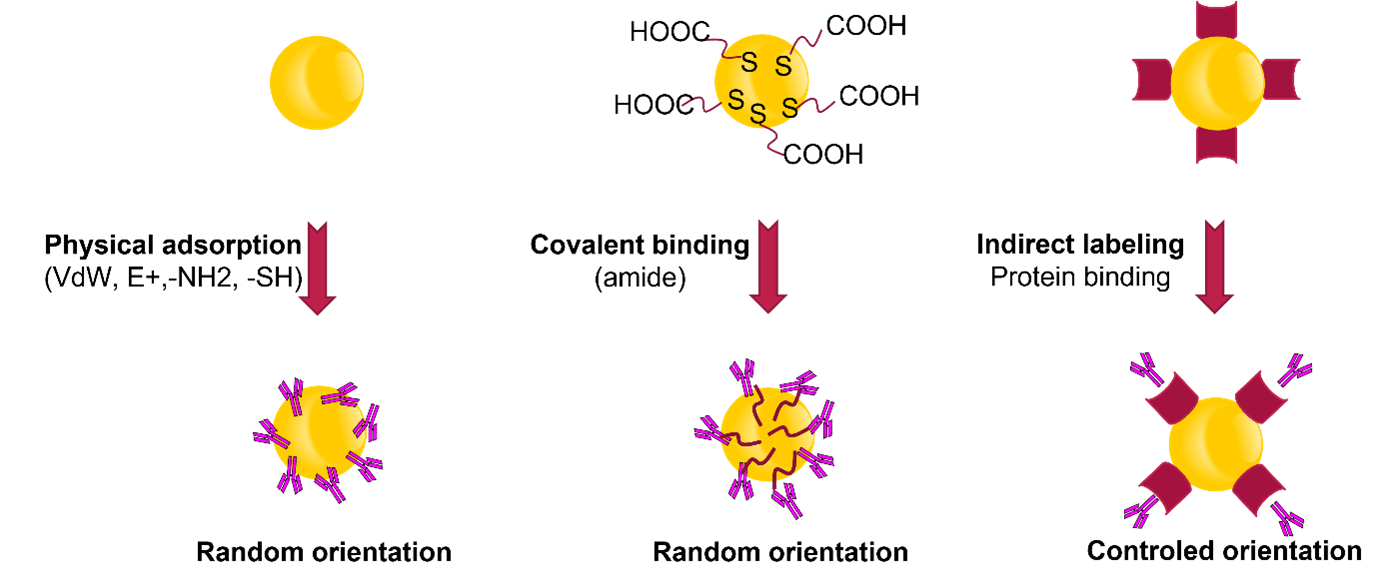
The simplest and often most effective method is through physical passive adsorption. This involves establishing multiple dative bonds and electrostatic interactions between the amines present on biomolecules and the gold surface, resulting in gold conjugates that possess the necessary stability and performance for use in lateral flow immunoassays. To benefit from best stability and performance, each antibody gold conjugate must be challenged in terms of conjugation pH and antibody concentration loaded on gold.
Alternatively, covalent binding can be chosen. In this case, the gold particles must first be functionalised with chemical groups on their surface, such as -COOH or -NH2. These specific chemical groups are typically introduced using a pegylated thiol that carries the desired chemical group at its distal end. In the presence of a carbodiimide (e.g., EDC), an amide bound is formed between the gold particles and the biomolecules. Drawing from our own experience, this method may prove advantageous, especially when dealing with non-biological samples that necessitate harsh analytical conditions. However, when it comes to biological samples, it does not present any benefits compared to physical adsorption, while simultaneously posing the challenge of being more costly and intricate to establish.
Another option is to utilise an indirect labeling method. For instance, antibodies can be indirectly labeled by employing anti-species antibodies, protein a or g, anti-biotin, or Streptavidin gold conjugates. This approach allows for the labeling of the primary antibody specific to the target. It can facilitate rapid screening of different primary antibodies, or the development of alpha or beta prototypes of tests. Moreover, this method is particularly useful for competitive tests. By using the unconjugated primary antibody and gold particles separately, one can easily modify the test line signal observed for negative samples without compromising the assay’s sensitivity.
Key Takeaways
Selecting the right critical raw materials, and the right partner to deliver these, is a pivotal step in the development of a commercially viable lateral flow immunoassay. Comparing different suppliers of gold nanoparticles and considering whether to produce gold conjugates in-house or outsource them are crucial decisions. With a clear understanding of the factors that influence the performance of gold nanoparticles and gold conjugates, you’ll be better equipped to make these critical decisions.
Abingdon Health is proud to work with innovative partners such as Nano Flow who have over 15 years of experience in manufacturing gold particles and gold conjugates and have successfully supplied more than 100 different antibody / protein gold conjugates to various industries. Nano Flow specialise in the development, scale-up, and production of antibody gold conjugates in volumes ranging from 50 millilitres to 20 litres and this approach dovetails with Abingdon’s established effective processes that ensure efficient and cost-effective development, scale-up, technical transfer, regulatory approval and high-volume manufacturing of lateral flow test products. Contact the Abingdon Health team to find out how we can help take your lateral flow test from R&D into robust manufacture and commercial success, and to discuss our value-added services such as regulatory and commercial support that minimise risk and streamline routes to market.

