LFA Design: Harnessing the Potential of Synthetic Peptides

How Do LFAs Work?
The Benefits of Lateral Flow Assays
How Peptides Are Used in LFAs
Why Use Peptides in LFA?
Points to Consider When Working with Peptides
Key Takeaways
In this new blog post, Sat Sandhu, Head of Peptide Chemistry at Alta Bioscience Ltd – a global leader in peptide synthesis for over 50 years – discusses the uses of peptides in LFAs, their advantages as well as best practices when choosing their sequences for successful synthesis and LFA development. Abingdon Health has proudly partnered with AltaBioscience – experts in Amino Acid Analysis, Peptide Synthesis and Protein Sequencing – over the last 15 years to deliver peptides for use in its Contract Development and Manufacturing (CDMO) lateral flow customer projects.
In recent years, lateral flow assays (LFAs) have shown enormous potential for point-of-care diagnostic testing, providing end-users with fast results and a clear colour readout when decision-making information is needed rapidly. Highly versatile, LFAs can detect a wide range of analytes at low concentration, and have found usage for prognostics, diagnostics, veterinary, environmental and food safety purposes.
Ongoing improvement in their sensitivity and specificity has led to a rapid increase in the number of applications, and at the heart of any LFA development is the critical selection of capture and detection reagents, as well as labels, in order to detect the analytes with specificity and sensitivity. In particular, synthetic peptides are particularly well suited for this purpose due to their high-specificity, customisability, stability, reproducibility and low development costs.
How Do LFAs Work?
Based on a similar principle as ELISA assay (enzyme-linked immunosorbent assay), LFAs harness the ability of biomolecules, such as antibodies, to bind specifically to a target antigen. As the sample flows through the nitrocellulose strip via capillary force, the analyte present in the sample will interact with the various molecules that have been confined in defined zones on the membrane. The main interactions typically occur at two reaction sites: firstly on the conjugation pad, where the target analyte will bind to nanoparticles conjugated with a detection molecule, such as an antibody. Subsequent interaction will then happen at the test line. LFAs are typically based on two possible formats: either sandwich (see Figure 1) or competitive (see Figure 2).
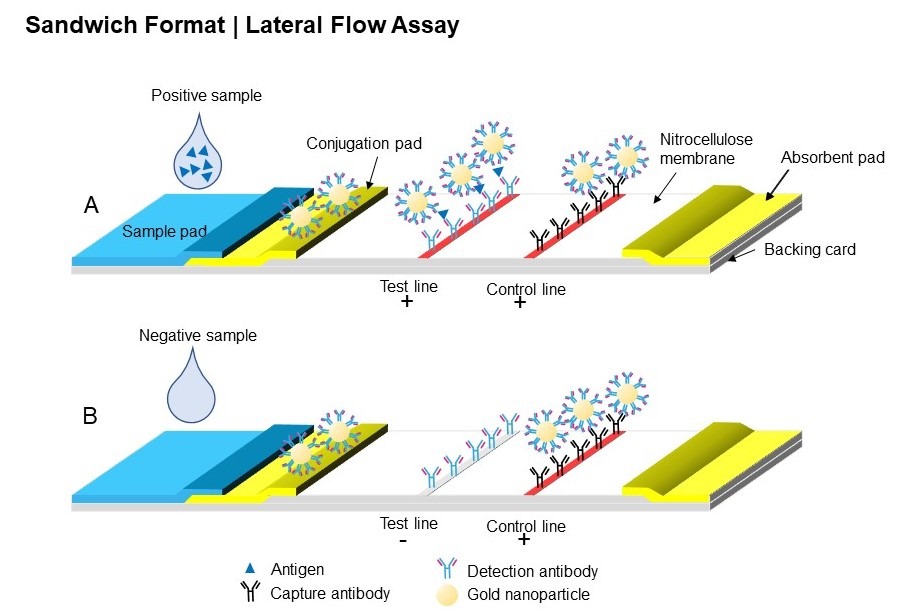
Figure 1: Schematic of sandwich format of LFA, (A) with and (B) without the analyte present in the sample.
In the case of a sandwich immunoassay (Figure 1), the analyte will also bind to a capture reagent. This will cause the nanoparticles to accumulate on the test line, resulting in the formation of a visible line (Figure 1, A).
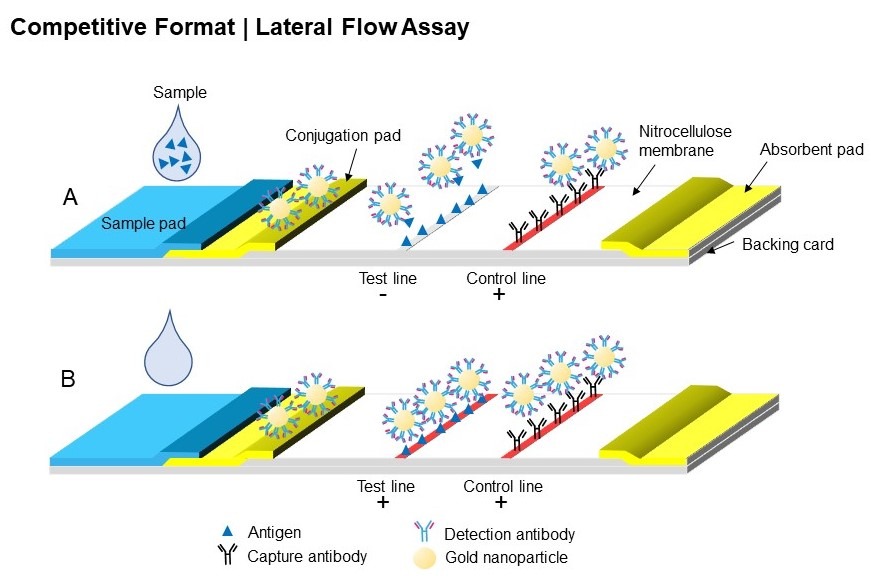
Figure 2: Schematic of competitive format of LFA, (A) with and (B) without the analyte present in the sample.
In the case of a competitive immunoassay (Figure 2), the analyte will compete against the nanoparticle conjugates for binding with the capture reagents on the test line. If the analyte is present in the sample, then no visible test line will be observed (Figure 2, A). Competitive LFAs are usually used with smaller analytes, or analytes that have only a single antigen.
Finally, to ensure enough sample has been added to the sample pad and the liquid has migrated through the entire length of the strip, a control line – where immobilised reagents are able to bind to the excess of nanoparticle conjugates – is usually added after the test line. A valid test will result in a positive line appearing.
The Benefits of Lateral Flow Assays
There are multiple advantages of LFAs. They are easy to use, portable, give fast results and can be used in a wide range of settings, whilst only requiring a small amount of sample. Additionally, they have the potential to detect a wide range of analytes due to their extreme versality of formats and varied choice for bio-recognition molecules, capture molecules and nanoparticle labels that can be modulated thanks to peptides.
How Peptides Are Used in LFAs
Peptides play an essential part in LFAs and depending on the analytes being tested for – antigens such as SARS-CoV-2 nucleoproteins or antibodies (IgG or IgM produced during an infection) for example – their usage will vary to meet the needs of the assay being developed.
In particular, they can be used:
Conjugated to nanoparticles to detect the analytes: Peptides are natural binding partners of antibodies, but have also been successfully employed as recognition molecules for bacteria using antimicrobial peptides in multiplex LFAs. Peptides can be covalently conjugated to latex beads, or bound to gold nanoparticles by electrostatic interactions, or covalent bonds either via chemisorption of free thiol groups (e.g., cysteine residues) or by conjugation to ligands via EDC (1-ethyl-3-carbodiimide hydrochloride) / NHS (N-hydroxy succinimide) chemistry.
- As gold nanoparticle caps: BSA (bovine serum albumin) or casein proteins are often used to cap gold nanoparticles, as well as short peptides (e.g., using CALNN, RRFPDD peptides) in order to prevent unspecific interactions, to improve solubility, or reduce nanoparticle aggregation.
- As antigen on the test line: Immobilisation of peptides onto nitrocellulose may be achieved by chemical conjugation; by indirect binding with Streptavidin using biotinylated peptides or by direct spotting onto the paper with the peptides adhering due to hydrophobic interactions.
- For antibody generation: Peptides can be used as immunogens to elicit monoclonal or polyclonal antibodies. Monoclonal antibodies bind a single epitope of their target and are usually preferred as capture antibody to ensure good specificity. Conversely polyclonal antibodies can bind to multiple epitopes and although this might increase the risk of non-specific binding, and lead to improper migration when conjugated to nanoparticles, their usage can improve the sensitivity of LFA. If using proteins as immunogen, epitope mapping studies using a library of overlapping peptides, as provided by AltaBioscience, is recommended to determine the epitope site of the antibody.
Why Use Peptides in LFA?
Peptides offers many advantages that can be harnessed when developing LFAs. In particular, they are useful tools due to their:
- Specificity: Synthetic peptides can be designed to mimic specific epitopes or regions of a target protein, ensuring high specificity in capturing the desired analyte. This specificity helps reduce false-positive or false-negative results.
- Customisability: Synthetic peptides can be easily tailored to match the desired target, allowing for customisation and flexibility in assay design. Peptide length, sequence, modifications, and conjugation options can be optimised to enhance assay performance.
- Stability: Synthetic peptides are often more stable than native proteins, which can be prone to degradation. Peptides can withstand harsh conditions during storage and transport, resulting in longer shelf life and improved assay reliability.
- Cost-Effectiveness: Compared to producing and purifying full-length proteins, synthesising peptides is generally more cost-effective. Peptide synthesis techniques have advanced significantly, making peptide-based assays a cost-efficient choice for many applications.
- Reproducibility: Synthetic peptides offer excellent batch-to-batch reproducibility, ensuring consistent assay performance over time. This reproducibility is crucial for assay manufacturing and large-scale production.
- Easy Characterisation: Synthetic peptides can be characterised from batch-to-batch, to confirm sequence, purity and net peptide content using Amino Acid Analysis in order to maintain the final concentration of the peptide when applying to lateral flow devices.
- Rapid Development: Peptide synthesis is a rapid process, allowing for quick assay development and optimisation. This speed is particularly advantageous for time-sensitive applications, such as diagnostic tests during outbreaks or epidemics.
- Safety: Synthetic peptides offer a safer alternative to working with live organisms or pathogenic materials that may be required for native protein production. They eliminate the risk of handling hazardous biological materials and increase overall laboratory safety.
Points to Consider When Working with Peptides
Solid-phase peptide synthesis is a widely used method for the chemical synthesis of peptides. The peptide chain is built one amino acid at a time in a repetitive cycle that includes an amine deprotection step, followed by a carboxylic activation step, and lastly a coupling step between the free amine moiety of the growing peptide on the solid support and the activated amino acid residue. Finally, the fully assembled peptide is deprotected, cleaved from the resin and purified using various techniques, such as reverse-phase High-Performance Liquid Chromatography (HPLC).
Although it is a well-established method, effective peptide design is crucial for successful synthesis and for achieving high sensitivity and specificity in LFAs. Therefore, there are key factors to consider when choosing a peptide sequence. These include:
- Selecting appropriate target-binding regions for antibody generation, or when multiplexing in order to allow specificity and avoid cross-reactivity.
- Choosing an adequate peptide length. It is recommended to limit the peptide sequence to less than 20 residues not only to cut costs, but also to increase yield and purity.
- Predicting peptide synthesis difficulty. Some peptides can be challenging, but these difficulties can be overcome, for instance, by decreasing the number of hydrophobic residues; by avoiding peptide sequences containing sections with several and juxtaposed Val, Ile, Tyr, Phe, Trp, Leu, Gln, and Thr; or by minimising the number of residues prone to oxidation.
- Immunogenicity of the peptide: peptides can suffer from low immunogenicity when used as immunogen for antibody generation. This can be overcome by using multiple antigenic peptides (a dendrimeric structure usually with a lysine core bearing multiple copies of the same peptide), or by forming peptide-protein conjugates with BSA or KLH (keyhole limpet hemocyanin) instead of using linear peptides in order to boost the immune response.
Key Takeaways
When designing LFAs, choosing the right combination of analytes, detection and capture molecules is crucial in order to develop new applications with high sensitivity and specificity. In particular, peptides have emerged as versatile tools in LFAs due to their flexibility in design and their many usages as detection or capture compounds; both for antibody generation, or to use as capping reagents to improve the properties of nanoparticle labels.
Working with an experienced partner like AltaBioscience, with over 50 years’ experience synthesising peptides for LFAs, will ensure successful synthesis of key peptide reagents for the development of new LFAs.
Decades of experience in taking tests from concept to commercialisation has led to Abingdon Health establishing effective processes that ensure efficient and cost-effective development, scale-up, technical transfer, regulatory approval and high-throughput manufacturing. Contact the Abingdon Health team to find out how we can help take your lateral flow test from R&D into robust manufacture and commercial success. Also, discuss our value-added services such as regulatory and commercial support that provides a de-risked and streamlined route to market. Abingdon Health is proud to partner with AltaBioscience.

