Vital Material Selection Considerations for Developing Your Lateral Flow Assay

Building Your Dream Test
Essential Components Deep Dive
Lateral Flow Tests of the Future
Key Takeaways
Developing precise, fit for purpose lateral flow devices is complex work. Not only must the science be robust, but the components that make up the device must be carefully selected and development processes rigorously delivered. In this blog Abingdon Health’s CTO, Nina Garrett, outlines what to look out for in vital material selection when developing your lateral flow assay.
Abingdon Health’s team has over 20 years’ experience in the lateral flow market and is a knowledge leader in the development, scale-up, transfer, manufacturing and regulatory approval of lateral flow products across a range of sectors. If you would like to understand more about the development, technical transfer, manufacturing and regulatory process and discuss any specific requirements don’t hesitate to contact Abingdon’s highly experienced team.
Building Your Dream Test
The benefits of the Lateral Flow Assay (LFA) format are undeniable. It is a simple, affordable technology which provides accurate and rapid results. Since inception, the use of LFAs has revolutionised fields from healthcare to food safety.
However, before you delve into developing your own LFA, considering what materials to use is paramount. Although the format and use of LFAs is relatively simple technology, developing the optimal combination of different materials and reagents to give consistent, reproducible and accurate results is key.
Essential Components Deep Dive
So, let’s explore the key ingredients – the vital materials – that can take your LFA from good to gold standard.
1. Conjugates: the Reagents That Label the Test Line
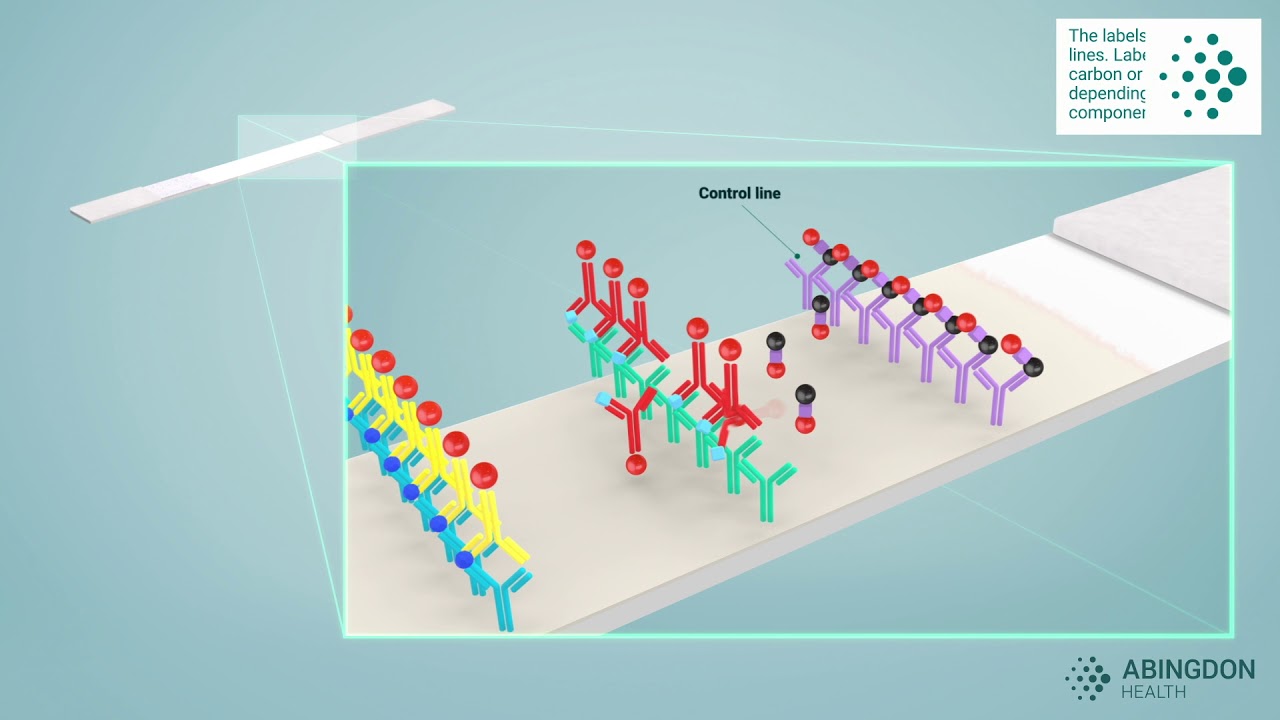
Conjugates are typically made from small nanoparticles, e.g., gold or latex, the size of which are in the nanometre scale. These small particles are linked to a specific molecule – often an antibody. In the case of an antibody conjugate, the antibody bound to the particle is specifically selected to bind to the target analyte that the lateral flow test is detecting. This video on our website explaining in more detail how lateral flow technology works, helps visualise the process, if you’d like to know more.
Some of the key features that are required of quality conjugates are outlined below.
- Specificity: This is the golden rule of conjugates. They must be highly specific to your target analyte, minimising false positives and ensuring accurate results.
- Consistency: the consistent performance of a conjugate will depend on the antibody that is being conjugated, as well as the process of conjugation. Selection of quality antibody reagents that perform consistently between lots, along with development of a robust conjugation methodology, are important to be confident that your reagent will give repeated performance in routine manufacture.
- Stability: Conjugates should be stable in storage both as a liquid in the manufacturing process and, also, as a dried reagent in the test. Stability of the conjugate material will ensure consistent performance and reliable results.
2. Sample Pad Selection and the Sample Matrix
The adaptability of lateral flow tests to different sample matrices has contributed to them being successfully adopted into everyday use in a wide range of industries and environments. When selecting sample pad materials there are a range of considerations that need to be thought through:
- How much sample will be available? Different pads are available that have different adsorption capacities.
- Is buffering of the sample required? E.g., will the pad need pre-treatment to manage high or low pH samples.
- Does the sample need to be filtered? E.g., does the sample pad need to remove particulates from the sample that may otherwise interfere in the test system.
- What is the sample matrix? Blood separation pads, for example, use a mixture of size exclusion and pre-treatment to separate the red blood cells from the whole blood sample matrix.
Fortunately, the materials available for selection for use in lateral flow devices have developed along with the overall technology and there are some fantastic options available to choose from to ensure optimal performance.
3. Cassettes or Housings: the Often-Overlooked Critical Parameter
Lateral flow tests can be presented in different physical forms such as test strip, or ‘dipstick’ only, or as a test strip that is presented in a cassette, also known as a ‘housing’. The housing plays several key roles in the function of the test strip, including:
- Protection of the test strip: particularly the nitrocellulose membrane, as it is delicate and can become damaged if not handled correctly. Also, when considering the environment of use, there are several considerations such as ‘splashing’ of the sample onto the test strip which would render the test ineffective.
- Promotion of flow of sample into the lateral flow test: the housing is designed to ensure that the different pad materials used in the test, such as the sample and conjugate pad, are correctly aligned to promote consistent flow of the sample into the lateral flow test. This in-turn ensures that the conjugate reagent is released uniformly, which results in a consistent test and control line forming.
- Direction for the end user is also included on the housing: markings can be put on the test cassette to direct the end user to the position of the test and control lines for ease of result interpretation, as well as guidance on where to add the sample to the test. Additionally, barcodes and other identifiers can be included on the housing, which is particularly relevant for quantitative tests as batch specific information can be included, as well as patient identification and stratification information. These identifiers can also enable smart and efficient data capture.
These considerations are also made when developing a ‘dipstick’ only version of a test and, often, printed cover-tapes are used to protect the test strip and provide direction for the end user, e.g., arrows are used to direct the end user, so they place the correct end of the test strip into the sample.
Lateral Flow Tests of the Future
At Abingdon Health, our extensive experience in lateral flow, commitment to continuous improvement, training & development, and drive to offer the best solutions to our customers means we remain at the forefront of new technology.
We’re regularly working with trusted partners to assess and, where quality criteria can be met, trial new components in our CDMO and CRO lateral flow test development projects. Label options are constantly evolving, as covered in detail recently by R&D Lead Scientist, Charlotte Howard, in a blog on how these innovations are driving growing use of lateral flow technology. Conjugate components too are being developed for best effect; see our previous blogs on gold nanoparticles and antibody conjugates, and peptides in analyte detection for more detail.
Innovations in housing manufacturing materials are also being worked on and are coming to the forefront of lateral flow development, including using recycled plastic and biodegradable materials for cassettes. For example, Abingdon recently invested in a partnership with Morrama Ltd, a leading UK-based certified B-Corp design agency, to develop a brand-new plastic free cassette to be manufactured in the UK. The Eco-Flo test cassette will utilise renewable plant fibre moulding technology that is biodegradable and reduces CO2 emissions by 80% compared to the equivalent single-use plastic and will be available from 2024; ensuring that the future of lateral flow is not only providing rapid, accurate results, but doing so in an environmentally sustainable way.
Key Takeaways
Remember, material selection is an intricate orchestration of a number of components working in perfect harmony. Each individual component influences the system in a different way and the optimal combination unlocks your LFA’s full potential.
It’s also essential to choose the right partner to work with who is up to date with current technology, works systematically backed by a robust QMS, and isn’t afraid to consider how innovative component options might deliver the best possible LFA product for your intended use.
Decades of experience in the development of LFAs at Abingdon Health, taking them from concept to commercialisation, has us well placed to develop your lateral flow test ensuring that the materials selected are appropriate for your test and allows you your desired end-product. We have established effective processes that ensures efficient and cost-effective development, technical transfer and scale-up of LFAs. Abingdon is ISO 9001 and ISO 13485 certified and adheres to Good Manufacturing Practice. This framework ensures that developed tests are manufactured in a consistent and robust manner batch in, batch out. Contact the Abingdon team to see how we can help take your test from R&D into reliable manufacture and commercial success. Also, discuss our value-added services such as regulatory and commercial support that provides a de-risked and streamlined route to market.

